This research highlight was originally published in NCI's 2021-2022 Annual Report.
Many promising nanomaterials appear to have qualities that could make them well-suited for use in anti-cancer therapies. The successful development of a candidate therapy can often be hampered, however, by a lack of understanding about its mechanism of action at the atomic level, which is very difficult to determine experimentally. Without a detailed appreciation of how a material may be acting to achieve a certain outcome, it is difficult to harness or further manipulate it for therapeutic purposes.
It is exactly this problem that Dr Judy Hart from UNSW Sydney sought to overcome as part of her 2021 National Computational Merit Allocation Scheme grant. Dr Hart used computing time on NCI’s Gadi supercomputer to simulate surface interactions and better understand the action of cerium oxide.
Cerium oxide has been used for decades in a range of industrial processes, including as an effective catalyst to increase the speed of critical chemical reactions. More recently, the potential use of cerium oxide nanoparticles in medicine is being explored, with the exciting possibility that they may be used to selectively target cancer cells.
Critically, cerium oxide has been observed to behave differently in particular cellular contexts. “In an alkali or basic environment, typical in healthy cells, cerium oxide acts beneficially, converting potentially damaging reactive oxygen species (ROS) to harmless water and oxygen,” Dr Hart explains. “However, in more acidic environments, typical in cancer cells, cerium oxide appears to act in the opposite way, actually driving the production of harmful ROS that could bring about cell death.”
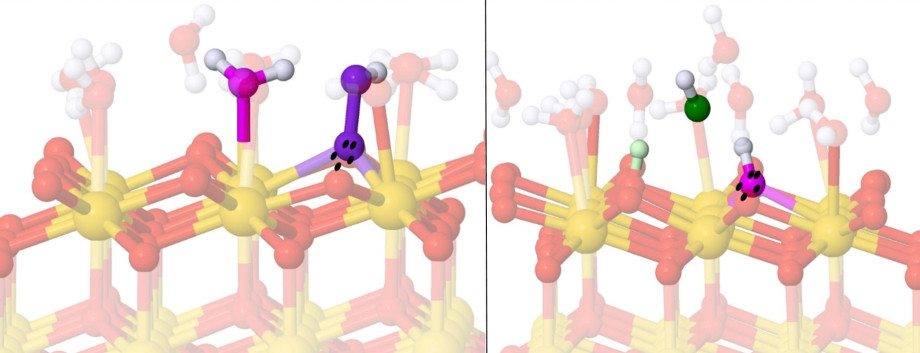
The possible therapeutic applications of such pH-dependent behaviour are clear. However, the lack of understanding of the atomic-scale mechanism at play has, to date, hampered exploration of its potential biomedical use.
To gain insights into how cerium oxide achieves its effects, Dr Hart’s research team used density functional theory calculations to study the interactions between the surface of cerium oxide and various molecules. Dr Hongyang (Jason) Ma, who worked as a PhD student on the project, says, “These computational approaches allow you to understand what is happening at the atomic scale, to ‘see’ the interactions between atoms and also control the system, modifying features to investigate how this changes surface chemistry and reactivity.” The team’s research findings provided evidence for the specific mechanism behind cerium oxide’s effects, an important step towards better control of the material’s pH-dependent behaviour and realisation of its cancer-treatment potential.
The future promises even greater outcomes from computational chemistry approaches. As the power and speed of supercomputers continue to be enhanced, the ability to model complex systems, more closely representative of reality, also increases. Dr Hart says, “The theory is actually ahead of the computational power available. Today we are able to model 100 atoms using fairly long-standing theory. While incredible insights are possible, this limits the scope of what you can do. In 10 years, with more powerful computing capacity and newer theories that have already been developed, we may be able to model 1000 atoms, and more effectively predict the real-world behaviour of materials.”